EY refers to the global organization, and may refer to one or more, of the member firms of Ernst & Young Global Limited, each of which is a separate legal entity. Ernst & Young Global Limited, a UK company limited by guarantee, does not provide services to clients.
How EY can help
-
Addressing an organization’s overarching end-to-end supply chain and operations strategy to grow, optimize and protect their operations.
Read more
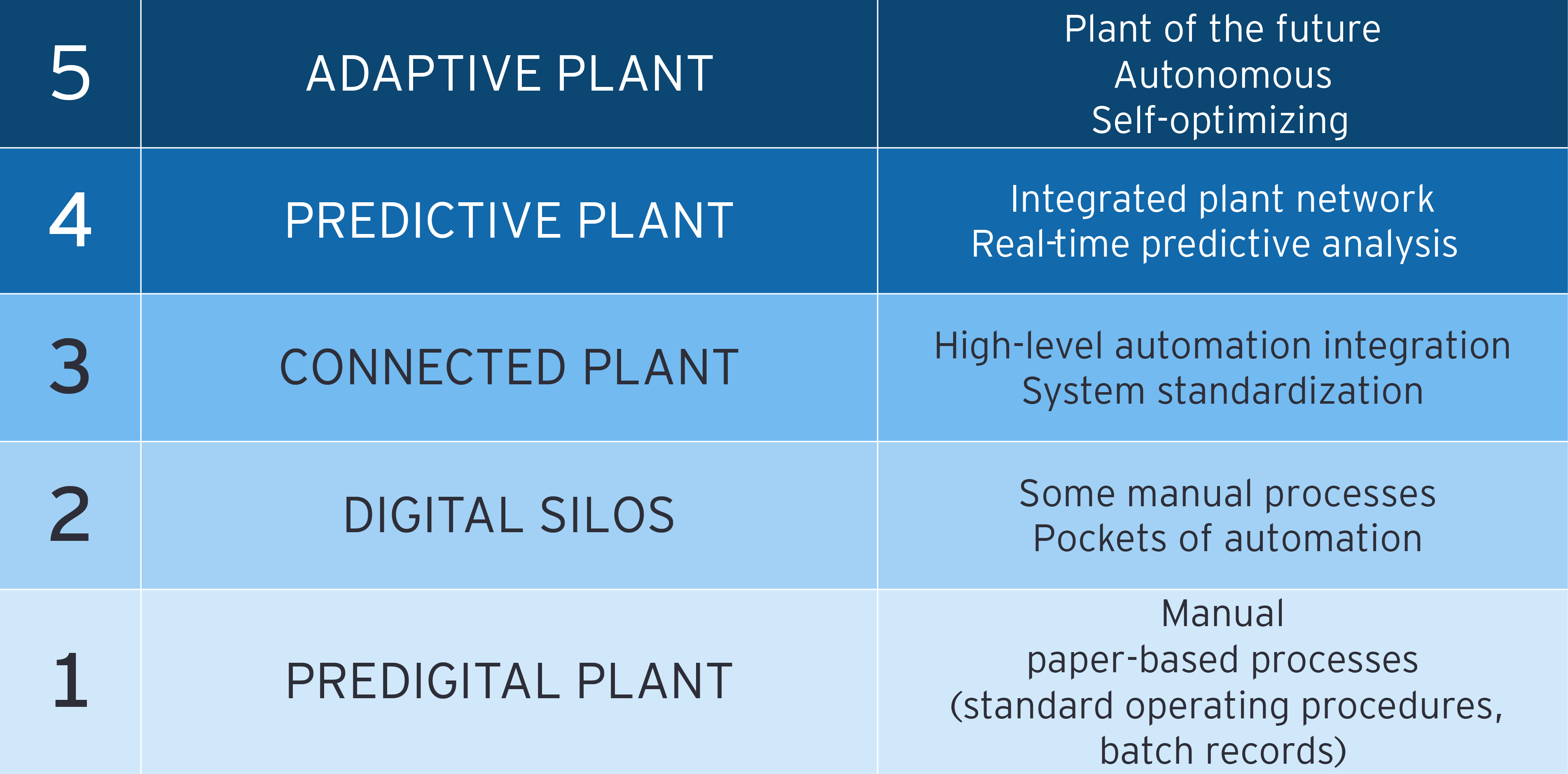
However, successful implementation of RTR is contingent upon several prerequisites, including robust manufacturing execution systems (MES), automation and the integration of multiple systems. By harnessing real-time data from various sources, manufacturers can achieve greater process transparency, optimize resource utilization and respond swiftly to market demands. Embracing digital transformation not only improves operational efficiency but also accelerates innovation, enabling the consistent delivery of safe, high-quality pharmaceuticals.
Real-time release testing is a quality release process that facilitates the near-instantaneous release of pharmaceutical drug products in batch and continuous manufacturing. Under the RTR approach, products must meet all in-process critical process parameters as well as any finished product testing requirements. While RTR does not alter the established release standards, it significantly enhances the speed and efficiency of the release process. Key considerations for implementing RTR include product quality, cost of manufacturing and speed of release.
How pharmaceutical manufacturing companies can benefit
The benefits of real-time release in the pharmaceutical industry are substantial and include the following: