EY refers to the global organization, and may refer to one or more, of the member firms of Ernst & Young Global Limited, each of which is a separate legal entity. Ernst & Young Global Limited, a UK company limited by guarantee, does not provide services to clients.

Explore the diversity of regulatory frameworks and market access landscape for digital therapeutics (DTx) worldwide, and identify approaches that less mature markets can employ to foster the growth and sustainability of DTx in Europe.
In brief
- Digital therapeutics (DTx) are transforming healthcare by delivering evidence-based interventions, with significant growth in Europe, particularly in Germany, France, the UK, and Belgium.
- Regulatory and market access for DTx vary globally; Germany leads with mature systems, while the US and other European nations are evolving, with room for improvement in reimbursement policies.
- Clear regulatory pathways, standardized evidence requirements, and collaborative stakeholder engagement are essential for integrating these innovations into healthcare systems and realize DTx's full potential.
Situation: The global landscape for digital therapeutics (DTx) is diverse, with established leaders such as Germany, the United Kingdom (UK), France, and Belgium, which have mature systems for DTx adoption. In contrast, other European and global markets show varying levels of maturity, affecting strategic planning for market entry and expansion of DTx solutions. This paper explores the evolution of digital therapeutics (DTx) regulations within Europe compared to the rest of the world, focusing on the integration of DTx into national healthcare systems through regulatory and reimbursement pathways.
Research and results: The research presents a comprehensive matrix assessing the maturity of various countries in adopting DTx solutions, based on the robustness of regulatory frameworks, pricing and reimbursement policies, and evidence requirements. It highlights the diverse approaches and readiness of different markets for DTx integration.
Lessons learned: The DTx industry is at a pivotal point, with some European nations leading in market access and setting benchmarks for regulatory frameworks, reimbursement policies, and evidence requirements. However, there is uneven advancement across Europe, calling for a collective effort to enhance healthcare systems in embracing DTx and stimulating industry innovation to ensure widespread patient access and secure the future of DTx. There will be transferable learnings across the continents that will help in creating a thriving global market for DTx products, considering a noteworthy progress in DTx policies in the United States (US), and Japan. A collaborative approach between DTx developers, regulators, and payers is crucial to establish a robust, pan-European DTx ecosystem that could serve as a model for global healthcare transformation.
1. Introduction to Digital Therapeutics:
The digital therapeutics (DTx) industry has experienced a surge in global enthusiasm in recent years, particularly following the COVID-19 pandemic and the advent of AI-enhanced digital health. Poised to transform treatment approaches and disease management, DTx represent a subset of digital health technologies that deliver evidence-based therapeutic interventions to patients through high-quality software programs or mobile apps. They aim to prevent, manage, or treat medical disorders or diseases, support patient adherence to treatment, enable behavior modification, and can be used independently or alongside other treatments.1 DTx differ from other digital health technologies in their therapeutic claims and the clinical evidence requirements. While digital health technologies include a broad range of tools such as health information systems, telehealth platforms, wearable devices, and wellness or lifestyle medical apps, DTx specifically target clinical outcomes and are regulated as medical interventions.2
The DTx sector has gained significant traction in Europe, with countries like Germany, France, the UK, and Belgium enhancing market access pathways, akin to pharmaceuticals, offering new care avenues for patients, healthcare providers, and policymakers. Since 2016, the DTx segment has been experiencing the fastest growth in the European health technology sector, expanding by approximately 9.0 times, in contrast to the US, where it has increased only 3.6 times.3 Valued at approximately USD 700 million in 2023, the European DTx market is expected to grow nearly five-fold by 2031, with a CAGR of 23.4%.4 Factors such as the rising prevalence of chronic diseases, the need for cost-effective and accessible health interventions, growing digital health policy support from local governments, and rapid technological advancement and adoption contribute to a fertile ecosystem for the growth of digital health companies and DTx solutions. This article aims to explore the current status of the regulatory and reimbursement landscape of DTx in Europe and compare the frameworks shaping the industry in Europe with key global markets such as the US, Canada, China, Japan, Australia, and Singapore.
2. Regulatory and Market Access Landscape of Digital Therapeutics in Europe: A Comparative Analysis:
DTx are typically classified under medical devices, with some jurisdictions establishing a standalone regulatory assessment pathway. The EU classifies DTx as medical devices, particularly software as a medical device (SaMD), requiring a CE (Conformité Européenne) marking to be marketed.1 The US FDA also categorizes DTx as SaMD, with a specific regulatory pathway.5 Japan's PMDA includes DTx in its medical device regulatory framework, requiring adherence to the same quality, safety, and efficacy standards as traditional medical devices.6
In this article, we assess the maturity of different countries in adopting DTx solutions by plotting a matrix based on the robustness of their regulatory framework, pricing and reimbursement policy, and evidence requirements (Figure 1). This matrix will serve as a valuable tool for stakeholders in the DTx industry to gauge market readiness for these innovative healthcare solutions (Figure 1). It also underscores the diversity in regulatory and market access environments across Europe and globally, which can inform strategic decision-making for market entry and expansion. A heat map featuring the maturity rating of regulatory policy, pricing and reimbursement environment, and evidence requirement policy is presented in Figure 2.
2.1 European landscape vs. global landscape:
Only a few European states have made progress in integrating market access policy for DTx into their respective healthcare systems.
Very high maturity markets: Germany stands out as the leader not only in Europe but also globally in terms of a well-established regulatory environment, a structured pricing and reimbursement policy, and clear evidence requirements for the assessment of DTx. Therefore, Germany can be classified as an extremely mature market that seems to offer advantageous conditions for both entering and maintaining a presence in the market.
High maturity markets: The UK, France, and Belgium are close followers of Germany, suggesting that they also have relatively mature systems in place for the adoption and integration of DTx. Among these, the UK and France have a better pricing and reimbursement scenario compared to Belgium. Belgium and the US share similar profiles, with solid regulatory frameworks and evidence requirements, but with room for improvement in reimbursement policies. Despite having a well-defined assessment pathway and a validation framework to approve the quality of DTx, none are currently reimbursed by the national health insurance in Belgium. The US has a strong regulatory environment, with established pathways for the assessment and approval of DTx but appears less advanced than Germany only from a pricing and reimbursement standpoint. Most of the US health plans, offered by managed care organizations (MCOs) or pharmacy benefit managers (PBMs), do not include a wide range of DTx — both prescription and non-prescription — in their standard benefits. Coverage of DTx in these plans is typically dependent on employer groups choosing to opt-in.
Moderate maturity markets: Mid-tier countries like Spain, Italy, and Finland are moderately mature, indicating frameworks and policies that are in the process of adapting to DTx. Japan, albeit scored moderately on this matrix, has taken steps to include DTx products in its national health insurance coverage, which is a significant move towards integrating these products into standard healthcare practice and enhancing their accessibility to patients. However, the lack of clarity around factors such as the extent of implementation of the evaluation framework, timeline for assessment and reimbursement, and the need for economic evidence ranked the country at a moderate maturity level.
Low and very low maturity markets: Lower maturity countries such as Estonia, Portugal, Norway, Denmark, and Ireland have room for substantial improvement in their regulatory and market access landscapes for DTx. On the global front, these markets are similar in maturity to Australia, China, Singapore, South Korea and Canada. Singapore has an innovation-friendly environment, with a regulatory framework that supports the development of DTx but lacks clarity on the value assessment parameters, reimbursement criteria, access channels and evidence requirements specifically for DTx. Countries such as Bulgaria, Croatia, Cyprus, Greece, Hungary, Malta, Poland, and Slovenia do not yet have established frameworks or policies for DTx.
2.2 Case studies:
To provide a nuanced understanding of how various DTx products have been successfully integrated into national healthcare systems, we present a series of case studies that navigate the distinct regulatory and reimbursement landscapes of Germany, the US, and the UK. In Germany and the US, the primary driver for the positive assessment of DTx products was clinical effectiveness. For example, in Germany, Kaia was assessed favorably due to its proven ability to improve outcomes in patients suffering from back pain7. Similarly, in the US, EndeavorRx, an app supporting attention-deficit/hyperactivity disorder (ADHD), received a green light for its robust clinical data demonstrating significant improvement in the primary endpoint evaluating the change in the test of variables of attention (TOVA) and Attention Performance Index (API)8. On the other hand, in the UK, cost-effectiveness was the key criterion that led to the endorsement of Sleepio9, which showcased not only clinical benefits but also a compelling economic case for its use in treating insomnia. These examples reflect the diverse criteria that can influence the assessment and adoption of DTx solutions across different healthcare systems.
3. Key insights:
Overall, the scores have indicated a diverse global landscape for DTx featuring prominent frontrunners, alongside a number of markets that are evolving their regulatory and market access structures. Manufacturers and regulators alike can use this information to guide strategic planning and policymaking to advance the field of DTx.
Albeit evolving as a leader in the market access of DTx, the pace of advancement in several European markets appears to have slowed down in recent years, with few markets implementing new policy or expanding the scope of existing regulatory frameworks. Germany, France, Belgium, and the UK continue as priority markets for DTx developers while other European countries continue to lag in their efforts to ramp up pricing and reimbursement policies that are essential to create a nurturing, integrated, and accessible DTx market across the continent. Below are specific policy recommendations and concrete steps that can be taken by less mature markets to stimulate innovation and competition within the industry, ultimately leading to the growth and sustainability of DTx in Europe.
- Establish clear regulatory pathways: Categorizing DTx under the broad umbrella of medical devices implies ambiguity for developers in terms of regulatory standards for the assessment of safety, quality, and efficacy. It is important to clearly define DTx and tailor a regulatory framework specifically encompassing guidelines on the evidence requirements and assessment criteria for approval in the local market context. The well-established regulatory frameworks in Germany and the US could potentially serve as best-in-class models for this exercise.
- Create pricing and reimbursement pathways: France, Belgium, and the UK could benefit from having a dedicated pricing policy, incentives, and reimbursement pathway for DTx similar to Germany. Upgrading from the existing ad hoc price negotiation framework would encourage more DTx options to venture into the market. In Belgium, introducing a fast-track assessment and reimbursement channel could be encouraging for DTx developers given that reaching the top of the mHealth validation pyramid is a time-consuming process. An example of simple adaptation to existing frameworks would be Japan’s model where the DTx assessment process has been integrated with standard therapeutic interventions and reimbursed as part of the national health insurance. Setting up special funds or payment channels to support DTx will also serve as stepping stones to eventually achieving a clear reimbursement policy.
- Standardize evidence requirements: In order to cultivate a supportive environment for DTx, it is imperative to establish a uniform set of standards for clinical evidence. While rigorous requirements, such as mandatory randomized controlled trials (RCTs), are crucial for ensuring the safety and efficacy of DTx solutions, the expectation to conduct RCTs for every product can be seen by developers as onerous and time-consuming. To balance the need for high-quality evidence with the practicalities faced by DTx developers, an evidence hierarchy system may be implemented. Under this system, lower-tier evidence, such as observational studies or real-world evidence, could lead to conditional approval or a reduced reimbursement rate, thereby allowing products to enter the market more readily. Conversely, DTx solutions backed by higher-tier evidence, including comprehensive RCT data, would be eligible for greater reimbursement. This tiered approach not only incentivizes the production of robust clinical data but also accelerates the availability of innovative treatments to patients, fostering a dynamic and progressive DTx landscape.
- Promote education, collaboration, and engagement among stakeholders: Fostering a symbiotic ecosystem for DTx necessitates the promotion of education, collaboration, and engagement among all key stakeholders. It is essential to encourage a spirit of cooperation among developers, healthcare providers, payers, and regulatory bodies to seamlessly weave DTx into the existing healthcare framework. These key stakeholders need to collaborate and establish a joint value proposition for DTx solutions, highlighting compelling reasons why DTx should be adopted and supported, the advantages they bring to patients, healthcare systems, and payers alike. At the same time, demonstrating that DTx solutions can deliver significant health benefits at a reasonable cost can help to make a strong case for the integration of DTx into healthcare systems. Local regulatory systems need to roll out comprehensive educational initiatives aimed at healthcare professionals aiming to enhance their familiarity with DTx, elucidating the advantages and practicalities of incorporating these therapies into patient care routines. Moreover, it is equally important to engage patients directly, bolstering their understanding and trust in DTx. By providing accessible information on the effectiveness and potential benefits of DTx, patients can be empowered to make informed decisions, thereby driving wider acceptance and adoption of these innovative treatments. Through these concerted efforts, we can ensure that DTx achieves its full potential in revolutionizing patient care.
4. Conclusion:
As we stand at the threshold of a new era in healthcare, the DTx industry has emerged as a transformative force, with select European nations paving the way in market access. Germany, France, Belgium, and the UK have established themselves as frontrunners, setting the standard for regulatory frameworks, reimbursement policies, and evidence requirements. Their strides in DTx market access serve as a beacon for others, demonstrating the profound impact of well-structured policy environments on healthcare innovation.
Despite these advancements, the journey towards a comprehensive and uniform DTx integration across Europe remains uneven. The disparity in regulatory and market access maturity calls for a concerted effort from slow adopters to fortify their healthcare systems, embrace DTx, and provide widespread patient access. This is not merely a matter of policy enhancement but a pivotal step towards stimulating industry innovation and securing a future where digital therapeutics are an integral part of patient care.
The path forward demands a synergistic alliance between DTx developers, regulators, and payers. It is through this collaborative spirit that policy gaps can be bridged, emerging markets can be harnessed, and a robust, pan-European DTx ecosystem can be cultivated. Such an ecosystem promises not only to revolutionize patient care within the continent but also to inspire and guide global healthcare transformation.
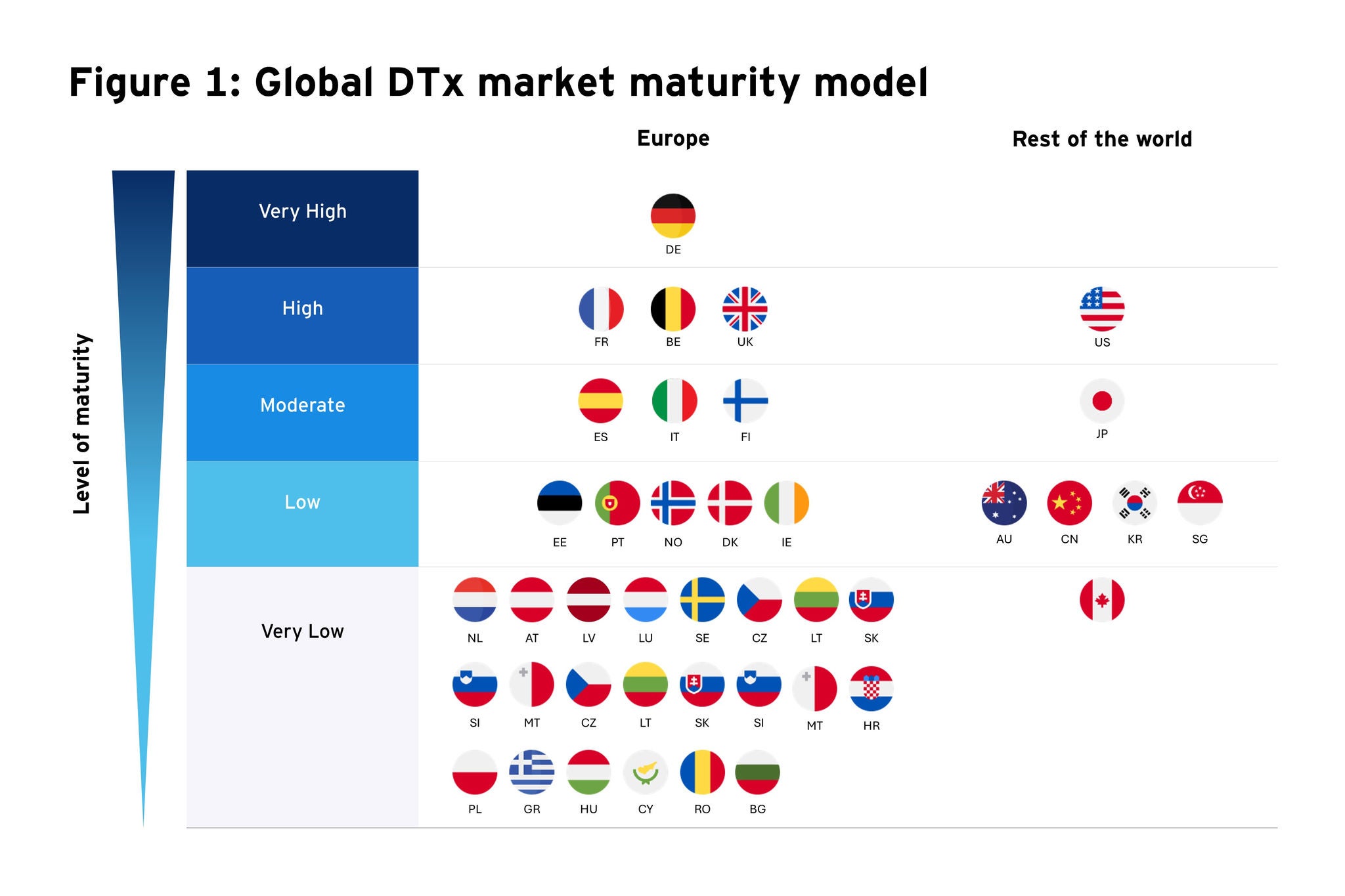
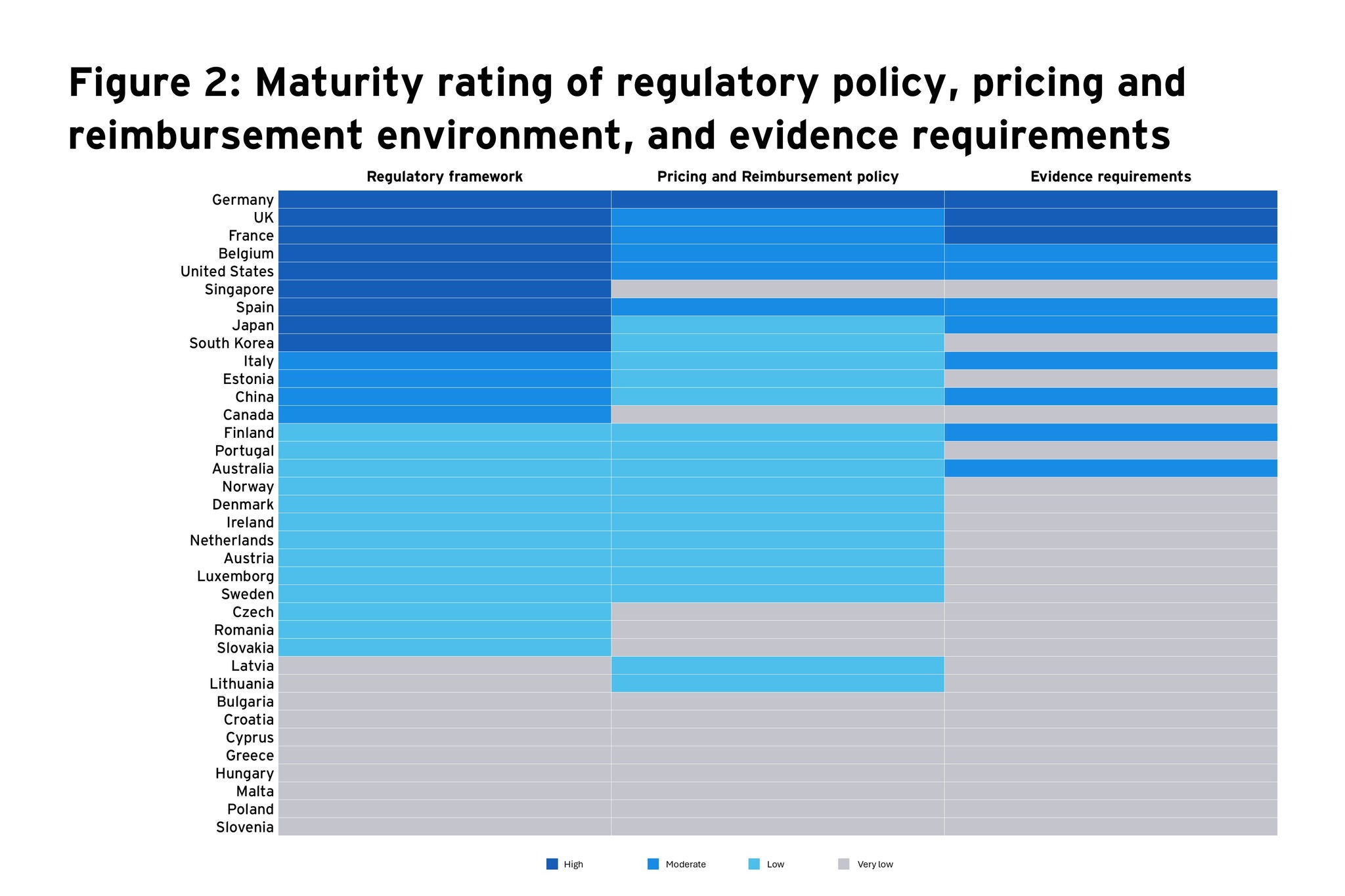
Table 1. Summary of decision rationale of DTx in Germany, the US, and the UK
CBT-I, cognitive behavioral therapy for insomnia; DTx, digital therapeutics; FDA, Food and Drug Administration; GP, general physician; MCID, minimal clinically important difference; NHS, National Health Service; NICE, National Institute for Healthcare and Excellence; RCT, randomized control trial; TOVA API, test of variables of attention – Attention Performance Index; VAS, visual analog scale.
Disclaimer: This publication contains information in summary form and is therefore intended for general guidance only. It is not intended to be a substitute for detailed research or the exercise of professional judgment. Member firms of the global EY organization cannot accept responsibility for loss to any person relying on this article.
Summary
At the dawn of a new healthcare epoch, DTx have become a pivotal influence, with certain European countries leading in adoption and accessibility. Countries like Germany, France, Belgium, and the UK are trailblazers, crafting exemplary policies and reimbursement models that inspire others and catalyze innovation. However, the progression toward uniform DTx adoption across Europe is fragmented, necessitating a unified effort from lagging countries to bolster their health care infrastructures and integrate DTx for broader patient benefit. Advancing this sector requires a united front among DTx manufacturers, regulatory bodies, and financial stakeholders to overcome policy discrepancies, nurture nascent markets, and establish a strong, Europe wide DTx network.



